Abstract
Introduction: Venous thromboembolism (VTE) is an important adverse event associated with immune checkpoint inhibitors (ICI) in cancer patients (12-15% annual rate). We have developed a murine model of venous thrombosis associated with ICI therapy (referred to as ICI-CAT). We found that mice bearing CT26 colon carcinoma treated with ICI developed larger thrombi following IVC occlusion compared with mice treated with IgG. ICI did not increase thrombus weight in mice without tumors. Tissue factor (TF) plays an important role in cancer-associated thrombosis (CAT) in patients treated with conventional therapies, and TF+ extracellular vesicles (EV) were associated with the development of VTE in pancreatic cancer. However, little is known about the impact of ICI on TF expression in patients treated with ICI. To address the underlying mechanism of ICI-associated thrombosis (IAT), we have examined the expression of TF in tumor and immune cells, as well as circulating EV, in our murine CT26 model.
Methods: A syngeneic colon carcinoma model (CT26) in BALB/c mice was utilized to evaluate ICI-CAT following inferior vena cava ligation (stasis). Tumor tissue was analyzed for TF expression by immunoblotting using an anti-TF antibody (ab189483, Abcam). Tumor tissue was also dissociated, and TF-positive cells were assessed by flow cytometry analysis with CD45+ and CD45- gating using antibodies for CD11b, Ly6C, Ly6G (BioLegend), and TF (R&D). EV were isolated from platelet poor plasma from ICI or control IgG-treated mice by centrifugation (20,000 x g). Procoagulant activity of EV TF was measured using a chromogenic substrate to detect FXa generation following incubation of EV with FVIIa in the presence or absence of an anti-TF antibody.
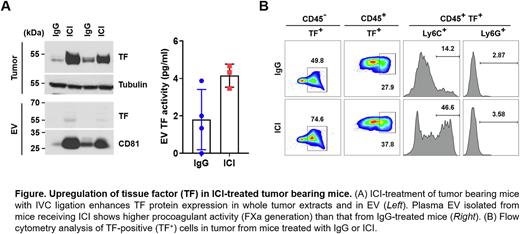
Results: Treatment of tumor-bearing mice with ICI markedly induced TF expression in tumors (Fig. A, Leftupper). Furthermore, levels of EV TF antigen (Fig. A, Left bottom) and EV TF activity (Fig. A, Right) were increased in mice treated with ICI. To explore the cellular origin of TF, we performed flow cytometry with dissociated tumor cells. A greater percentage of TF-positive cells in both the CD45+ (37.8%) and CD45- (74.6%) populations were detected in tumors from ICI treated mice compared to those treated with control IgG (27.9% and 49.8%, respectively), suggesting both tumor and tumor-infiltrating immune cells contribute to the larger thrombi observed in ICI-treated mice (Fig. B). Further analysis of CD45+ TF+ cells by CD11b gating (data not shown) revealed that a TF expression in a monocyte population (CD11b+ Ly6C+) was increased 3-fold by ICI (46.6%) compared to control IgG (14.2%) (Fig. C). We also found that TF expression was increased in cultured CT26 cells in response to interferon-g, but not in response to anti-PD-1 and anti-CTLA-4 antibodies, suggesting that ICIs do not directly upregulate TF and that more complex cellular interactions and inflammatory changes may be involved.
Conclusions: ICI treatment of tumor-bearing mice results in increased tumor TF expression and EV TF expression. TF was also upregulated in immune cells in the tumor microenvironment after ICI treatment, including both tumor/stromal cells (CD45-), and tumor-associated monocytes and neutrophils (CD45+). These results suggest that multiple underlying mechanisms and diverse cellular expression underlie TF expression in this model, and that these processes many contribute to the larger thrombi observed in ICI-treated mice.
Disclosures
Khorana:Anthos: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.